MR. BMJ
Senior Moderators
Staff Member
- Sep 21, 2011
- 2,520
- 2,575
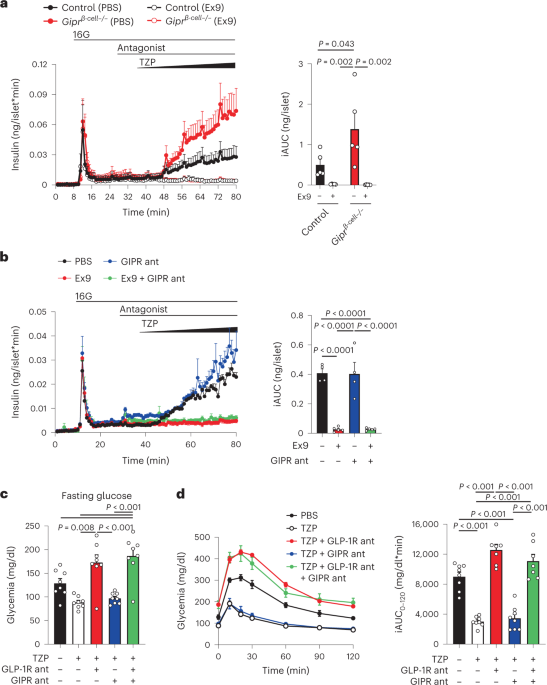
We have one for Semaglutide, so I figured we might as well throw one up for tirzepatide as well. They are both GLP-1 agonists, but tirzepatide has a dual-action in that it also acts as a glucose-dependent insulinotropic polypeptide (GIP) drug.
Here is a newer meta-analysis below. Feel free to add experiences if yuou have used this drug, as well as any future studies done on it. There are a few I may have already posted here to TID, and I know there were some we had over at WCBB. If I get time, i'll add those in here. Anyway......,
*************************************************************
Full text of the study in link:

 www.ncbi.nlm.nih.gov
www.ncbi.nlm.nih.gov
Meta-Analysis. PLoS One. 2023 May 4;18(5):e0285197.
Methods: Cochrane Library, PubMed, Embase, Clinical Trials, and Web of Science were searched from inception to October 5, 2022. All randomized controlled trials (RCTs) were included. The odds ratio (OR) was calculated using fixed-effects or random-effects models by Review Manager 5.3 software.
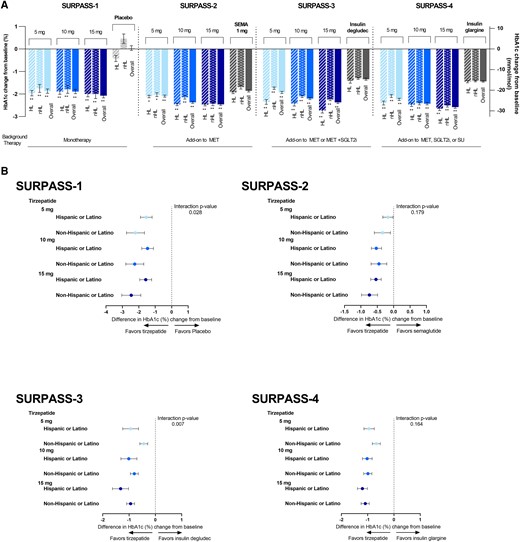
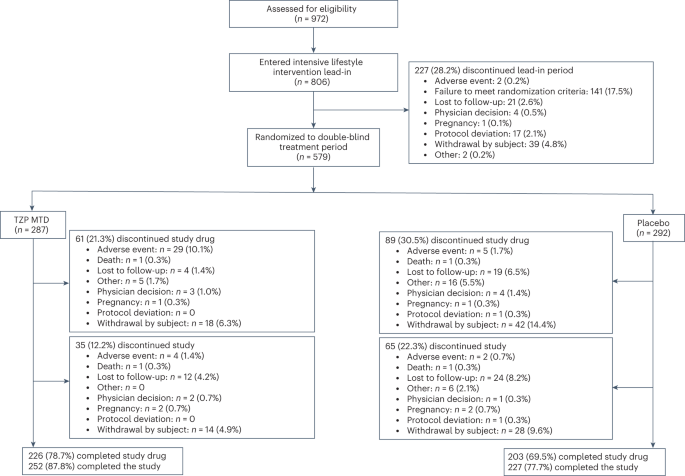
Results: In total, ten studies (12 reports) involving 9,873 patients were identified. A significant loss body weight in the tirzepatide group versus the placebo by -9.81 kg (95% CI (-12.09, -7.52), GLP-1 RAs by -1.05 kg (95% CI (-1.48, -0.63), and insulin by -1.93 kg (95% CI (-2.81, -1.05), respectively. In sub-analysis, the body weight of patients was significantly reduced in three tirzepatide doses (5 mg, 10 mg, and 15 mg) when compared with those of the placebo/GLP-1 RA/insulin. In terms of safety, the incidence of any adverse events and adverse events leading to study drug discontinuation was higher in the tirzepatide group, but the incidence of serious adverse events and hypoglycaemia was lower. Additionally, the gastrointestinal adverse events (including diarrhea, nausea, vomiting and decreased appetite) of tirzepatide were higher than those of placebo/basal insulin, but similar to GLP-1 RAs.
Conclusion: In conclusion, tirzeptide can significantly reduce the weight of T2DM and patient with obesity, and it is a potential therapeutic regimen for weight-loss, but we need to be vigilant about its gastrointestinal reaction.
Here is a newer meta-analysis below. Feel free to add experiences if yuou have used this drug, as well as any future studies done on it. There are a few I may have already posted here to TID, and I know there were some we had over at WCBB. If I get time, i'll add those in here. Anyway......,
*************************************************************
Full text of the study in link:

Weight loss efficiency and safety of tirzepatide: A Systematic review
Tirzeptide is a novel glucagon-like peptide-1 receptor (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) drug, which shows good efficiency for weight loss. Therefore, we aim to investigate the efficacy and safety of tirzepatide for weight ...
Meta-Analysis. PLoS One. 2023 May 4;18(5):e0285197.
Weight loss efficiency and safety of tirzepatide: A Systematic review
Fei Lin 1 2, Bin Yu 3, Baodong Ling 4, Guangyao Lv 5, Huijun Shang 6, Xia Zhao 1 2, Xiaoling Jie 1 2, Jing Chen 1 2, Yan Li 1 2Abstract
Objective: Tirzeptide is a novel glucagon-like peptide-1 receptor (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) drug, which shows good efficiency for weight loss. Therefore, we aim to investigate the efficacy and safety of tirzepatide for weight loss in type 2 diabetes mellitus (T2DM) and obesity patients in this meta-analysis study.Methods: Cochrane Library, PubMed, Embase, Clinical Trials, and Web of Science were searched from inception to October 5, 2022. All randomized controlled trials (RCTs) were included. The odds ratio (OR) was calculated using fixed-effects or random-effects models by Review Manager 5.3 software.
Results: In total, ten studies (12 reports) involving 9,873 patients were identified. A significant loss body weight in the tirzepatide group versus the placebo by -9.81 kg (95% CI (-12.09, -7.52), GLP-1 RAs by -1.05 kg (95% CI (-1.48, -0.63), and insulin by -1.93 kg (95% CI (-2.81, -1.05), respectively. In sub-analysis, the body weight of patients was significantly reduced in three tirzepatide doses (5 mg, 10 mg, and 15 mg) when compared with those of the placebo/GLP-1 RA/insulin. In terms of safety, the incidence of any adverse events and adverse events leading to study drug discontinuation was higher in the tirzepatide group, but the incidence of serious adverse events and hypoglycaemia was lower. Additionally, the gastrointestinal adverse events (including diarrhea, nausea, vomiting and decreased appetite) of tirzepatide were higher than those of placebo/basal insulin, but similar to GLP-1 RAs.
Conclusion: In conclusion, tirzeptide can significantly reduce the weight of T2DM and patient with obesity, and it is a potential therapeutic regimen for weight-loss, but we need to be vigilant about its gastrointestinal reaction.